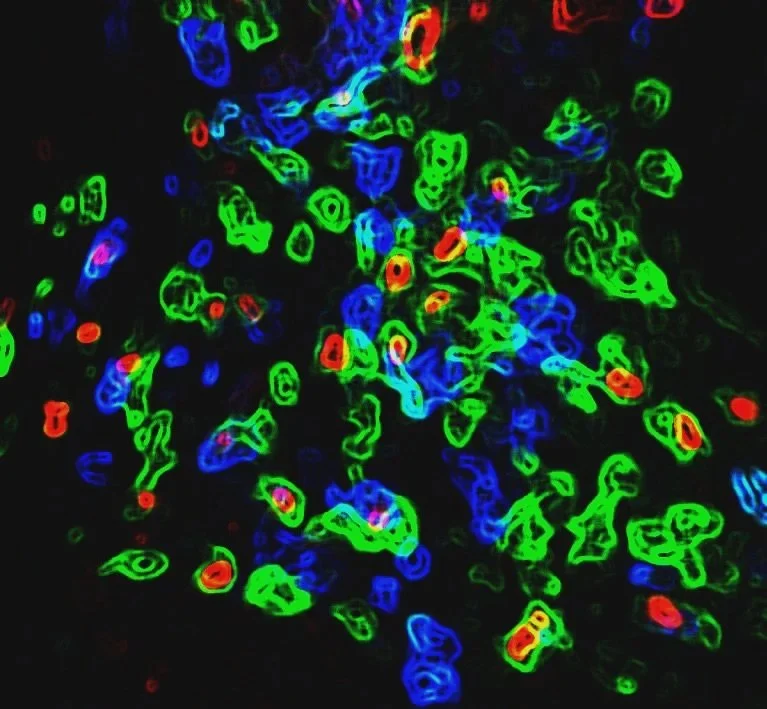
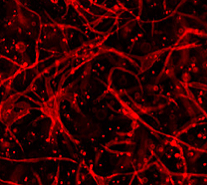
Learn more about the research done by the NorCal CoE team
The research undertaken by the team of innovative and collaborative investigators at the BT1D Northern California Center of Excellence (NorCal CoE) is divided into three main thematic projects: basic research on T1D pathogenesis, translational work to develop cell therapies to promote immune tolerance, and cellular engineering to advance beta cell replacement therapy.
Project 1 focuses on investigating the mechanisms of autoimmunity. It aims to dissect the mechanisms of extreme phenotypes of T1D, examine the role of islet stromal cells in T1D pathogenesis, and model T1D pathogenesis using human iPSCs.
Project 2 focuses on immunotherapy for T1D. The goal is to engineer immune tolerance in T1D by developing Treg cell therapy to enable lasting immune tolerance in T1D, investigating and overcoming HSC engraftment resistance in NOD mice, and modulating immune responses using tolerogenic dendritic cells by investigating the role of EpoR signaling on type 1 dendritic cells (cDC1) in the induction of immune tolerance to islet cell transplants.
Project 3 aims to improve post-transplantation beta-cell function by providing and enhancing vascularization. The project aims to characterize the islet vascular niche, enhance islet grafts with human stem cell-derived endothelial cells, and investigate the molecular control of vascular stability after transplant.
PROJECT 1
Probing T1D Pathogenesis
-
Project 1 aims to dissect the autoimmune pathogenesis of Type 1 Diabetes (T1D). We will investigate mutations identified in humans with monogenic T1D using mouse models. We will explore the role of islet stromal cells in regulating the tissue microenvironment, particularly their influence on the balance between pro-repair and pro-inflammatory responses. We will construct models of T1D using human stem cell-derived systems to enable testing of novel therapies.
-
PROJECT 2
Engineering Immune Tolerance
-
Project 2 focuses on translational research aimed at developing immunotherapies. We will design strategies for engineering regulatory T cells (Tregs) to support combination immunotherapies that both suppress pathogenic effector cells and enhance Treg function. In parallel, we will continue optimizing approaches to achieve robust hematopoietic chimerism using low-toxicity preconditioning. We will also investigate the novel role of EpoR in cDC1 induction of Tregs and develop strategies to harness the tolerogenic potential of this pathway to enable islet transplant without immunosuppresion.
-
PROJECT 3
Building Vascular Niche-Enhanced Islets
-
Project 3 aims to enhance beta cell function following transplantation through the incorporation of vascular cells. We will characterize the molecular features of islet vascular niche cells and generate stem cell-derived vascular cells that replicate the properties of native islet vasculature. Additionally, we will investigate the molecular mechanisms that support vascular stability of transplanted parathyroid gland and develop parathyroid mimetics to preserve intra-islet vascular after transplant.
-